Researchers at Scripps Research have made a significant breakthrough in understanding transthyretin amyloidosis (ATTR). A progressive and often fatal disease caused by the misfolding of the transthyretin protein. Their findings, published in Nature Structural & Molecular Biology on January 22, 2025, reveal that it has unexpected structural asymmetry, possibly contributing to its instability and disease-causing clumping.
This discovery could lead to more effective treatments for ATTR, which affects up to one in four men over 80 and causes shortness of breath, dizziness, and nerve damage.
New Insights Into Transthyretin, a Long-Studied Protein
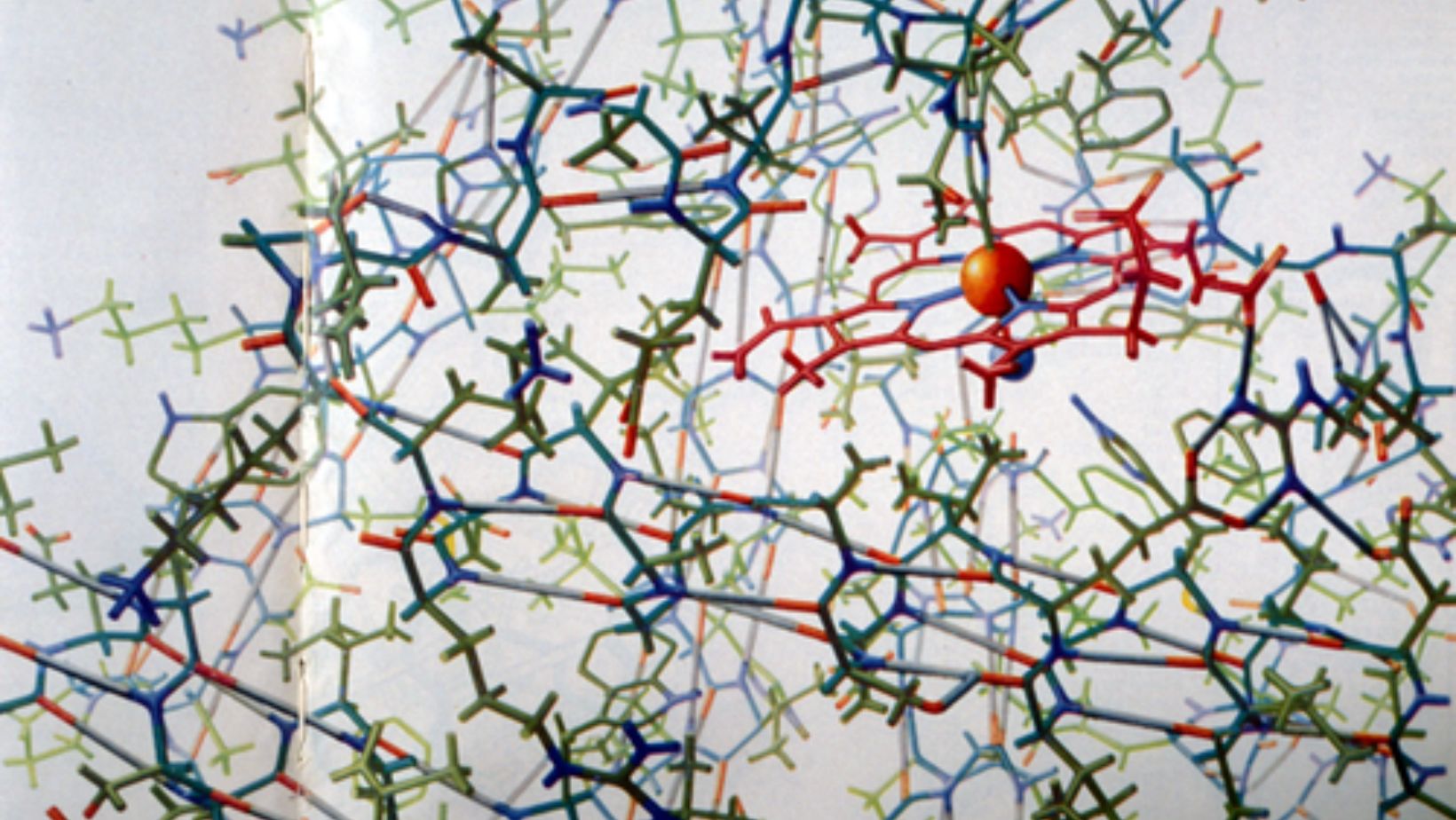
Transthyretin is a transport protein that carries hormones through the blood and spinal fluid. When properly folded, it functions normally, but when it misfolds, it forms harmful clumps in the heart and nervous system, leading to ATTR. Until now, scientists believed that transthyretin had two identical hormone-binding sites, but new research shows that these sites are structurally different.
“We’ve unveiled a molecular complexity hidden from researchers for decades, which enables us to design better medicines to stabilize transthyretin,” said Gabriel Lander, PhD, professor at Scripps Research and co-senior author of the study.
This newfound asymmetry could explain how transthyretin misfolds and why certain drugs stabilize it more effectively than others.
Advanced Imaging Techniques Reveal Hidden Instability
Researchers often use X-ray crystallography to study transthyretin’s structure, which forces proteins into large, repetitive crystal structures that may not accurately reflect how they behave in the body. Another method, cryo-electron microscopy (cryo-EM), provides a more natural view but has challenges—small proteins like transthyretin tend to become unstable during imaging.
To overcome this, Lander’s team developed a graphene-coated grid that allowed transthyretin molecules to maintain their natural shape while being imaged. This breakthrough enabled capturing transthyretin’s natural movement, which researchers likened to “molecular breathing.”
“Getting the surface chemistry right is crucial for this type of study,” said Benjamin Basanta, PhD, first author and former research associate at Scripps Research.
A Path to More Effective Treatments

One of the key findings from this research was how the ATTR drug tafamidis, developed by Jeffery Kelly, PhD, interacts with it. The team found that tafamidis binds to it and stabilizes it, preventing the misfolding process that leads to disease.
Beyond ATTR, the team believes their graphene grid method could be used to study other small, unstable proteins, including amyloid-beta peptides, which play a key role in Alzheimer’s disease.
“The methodologies we’ve developed have opened new avenues of treatment that could one day protect patients from not just TTR amyloidosis but other amyloid diseases as well,” said Lander.
This research was supported by the National Institutes of Health and the George E. Hewitt Foundation for Medical Research. With these new insights, scientists hope to develop targeted drugs that prevent protein misfolding, improving treatments for ATTR and other neurodegenerative diseases.
Reference: Benjamin Basanta, Karina Nugroho, Nicholas L. Yan, Gabriel M. Kline, Evan T. Powers, Felix J. Tsai, Mengyu Wu, Althea Hansel-Harris, Jason S. Chen, Stefano Forli, Jeffrey W. Kelly, Gabriel C. Lander. The conformational landscape of human transthyretin revealed by cryo-EM. Nature Structural & Molecular Biology, 2025.

