Researchers at the University of Michigan have identified a promising alternative treatment for cutaneous lupus erythematosus (CLE). A skin condition commonly associated with systemic lupus erythematosus. The study suggests that a topical antibiotic, mupirocin, could help reduce inflammation in lupus-related skin rashes. This offers a potential non-pill option for patients already managing multiple medications.
Cutaneous Lupus: A Challenging Condition
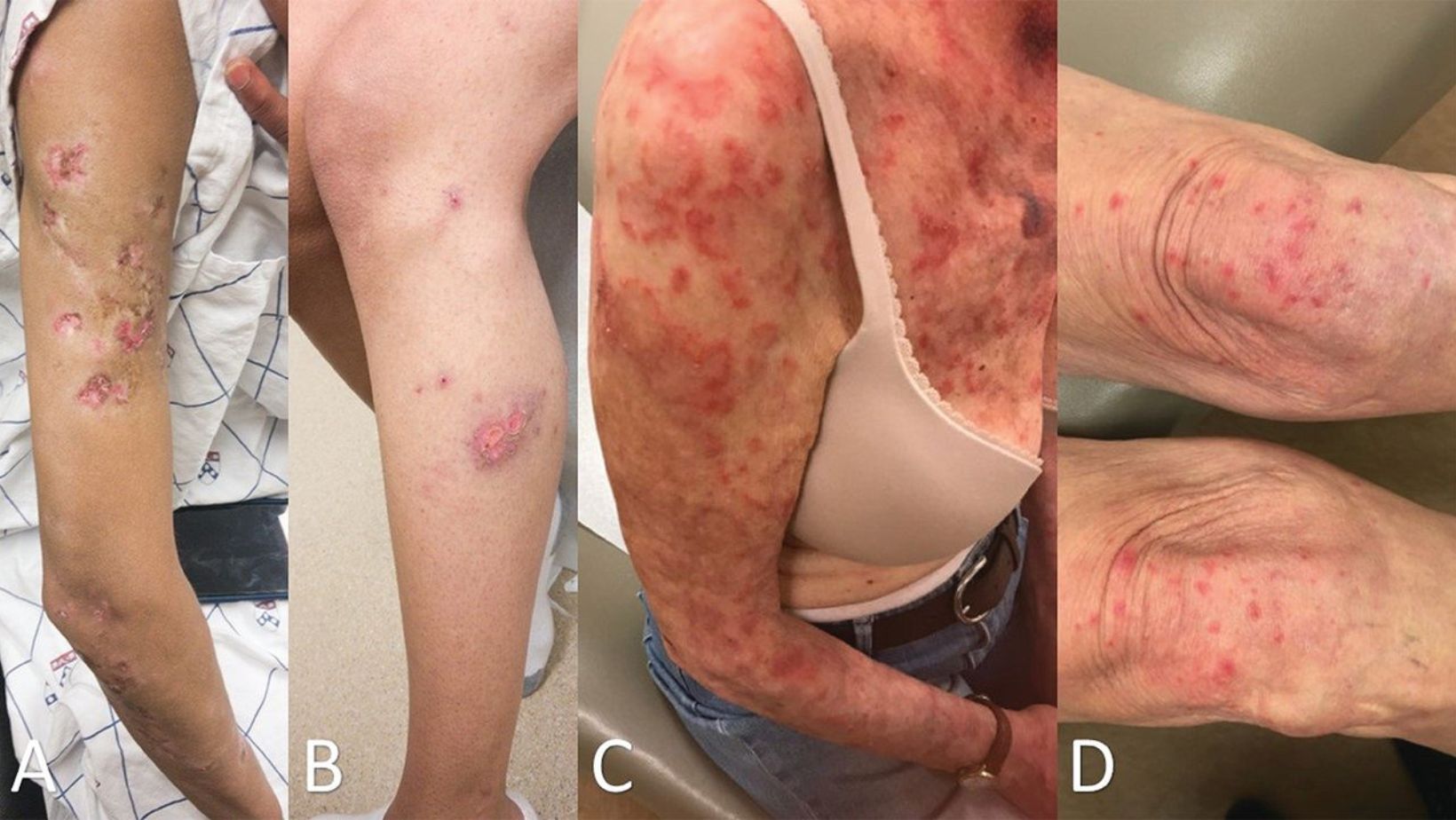
Cutaneous lupus erythematosus causes rashes, hair loss, and scarring, primarily affecting the face and scalp. These symptoms result from the immune system mistakenly attacking the body’s tissues, leading to chronic inflammation.
Standard treatments typically involve immunosuppressants and biological drugs to control inflammation. While effective, these medications comes with side effects, and lupus patients are already taking multiple prescriptions to manage their condition. This has led researchers to explore alternative approaches, including topical treatments.
Targeting Staph Bacteria to Reduce Inflammation in Skin Rashes
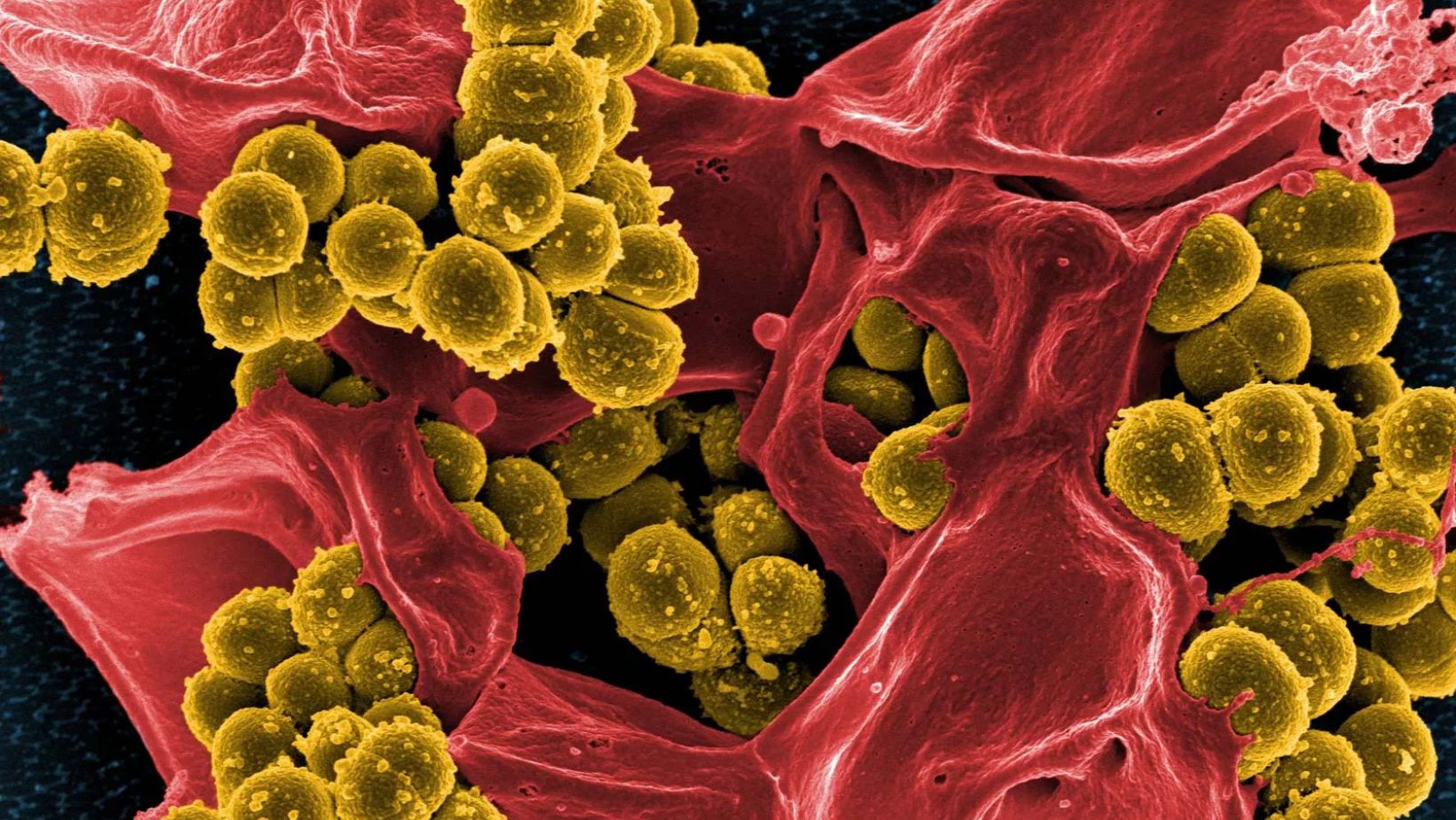
The study, led by Dr. J. Michelle Kahlenberg, was based on her earlier discovery that lupus skin rashes are often colonized by Staphylococcus aureus, a common bacteria known to trigger inflammation. Mupirocin is a prescription antibiotic designed to kill S. aureus, making it a potential tool for managing CLE.
In the trial, lupus patients experiencing active skin flares were randomly assigned to apply either mupirocin or an inactive petrolatum jelly to their rashes. Researchers collected skin and nasal swab samples before and after seven days of treatment to analyze bacterial levels and inflammatory markers.
The results showed a significant reduction in S. aureus in the mupirocin-treated group. More importantly, this bacterial decrease was accompanied by lower inflammatory signals, including a reduction in interferon-driven gene expression—key contributors to lupus-related skin inflammation.
“In addition to decreasing the inflammation by decreasing Staphylococcus aureus, the mupirocin treatment also lowered skin monocyte levels, which are important in driving cutaneous lupus,” Kahlenberg explained.
A Step Toward New Treatment Options
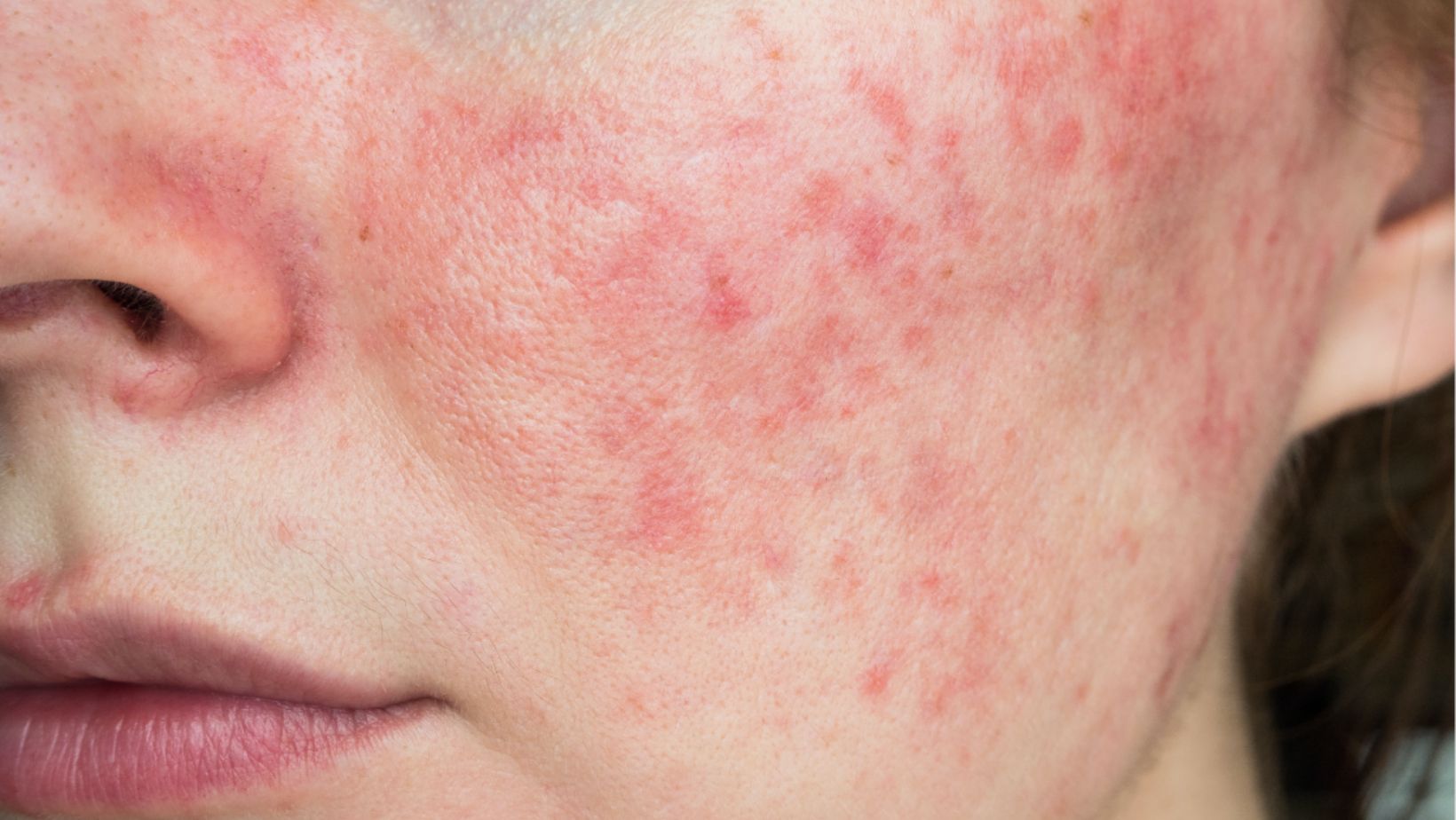
While the findings are encouraging, Kahlenberg emphasized that the study was not designed to determine whether mupirocin could visibly cause lupus rashes. Further research with more extensive clinical trials is needed to confirm whether topical antibiotics can relieve CLE symptoms.
“Additional larger studies are needed to determine whether topical antibiotics will help make rashes disappear,” Kahlenberg said. “However, this is an exciting first step to show that there may be additional treatments that can improve inflammation beyond our usual immunosuppressant and biologic drugs.”
If future research supports topical antibiotics for lupus skin conditions, this could offer a simple, targeted approach to managing inflammation without adding to the medication burden for lupus patients.
Reference: Lisa Abernathy‐Close, Joseph Mears, Allison C. Billi, Sirisha Sirobhushanam, Celine Berthier, Annie Lu, Zeran Zhang, Amy Hurst, Johann E. Gudjonsson, J. Michelle Kahlenberg. Topical Mupirocin Treatment Reduces Interferon and Myeloid Signatures in Cutaneous Lupus Erythematous Lesions Through Targeting of Staphyloccal Species. Arthritis & Rheumatology, 2025.

