Scientists at Tonix Pharmaceuticals in Frederick, Maryland, have developed an experimental vaccine that could improve protection against smallpox and pox while reducing side effects. This uses the horsepox virus, aims to combine the strong immunity of traditional live-virus vaccines with the improved safety of modern replication-deficient of it are all in a single dose.
A Safer Alternative to Existing Vaccines

Currently, two types of smallpox vaccines are available. One is a single-dose of it containing live vaccinia virus, which provides strong immunity but carries a risk of serious side effects. The other, a two-dose of this made with a replication-deficient virus, is safer but requires multiple doses for complete protection. The new experimental vaccine from Tonix Pharmaceuticals seeks to bridge this gap by offering a safer, single-dose alternative.
According to a study published this week in mSphere, the horsebox virus used in the experimental vaccine is significantly more attenuated—meaning it is weakened and less likely to cause a systemic infection—than the vaccinia virus in the FDA-approved single-dose of it. Researchers found that the horsebox virus was up to 1,000 times more attenuated than vaccinia virus strains, potentially making it a safer option.
“We are trying to go for the immunity of the old vaccines and safety profile of the new ones, and so far, the data are auspicious,” said virologist Farooq Nasar, Ph.D., senior author of the study.
Addressing the Ongoing Threat of Mpox
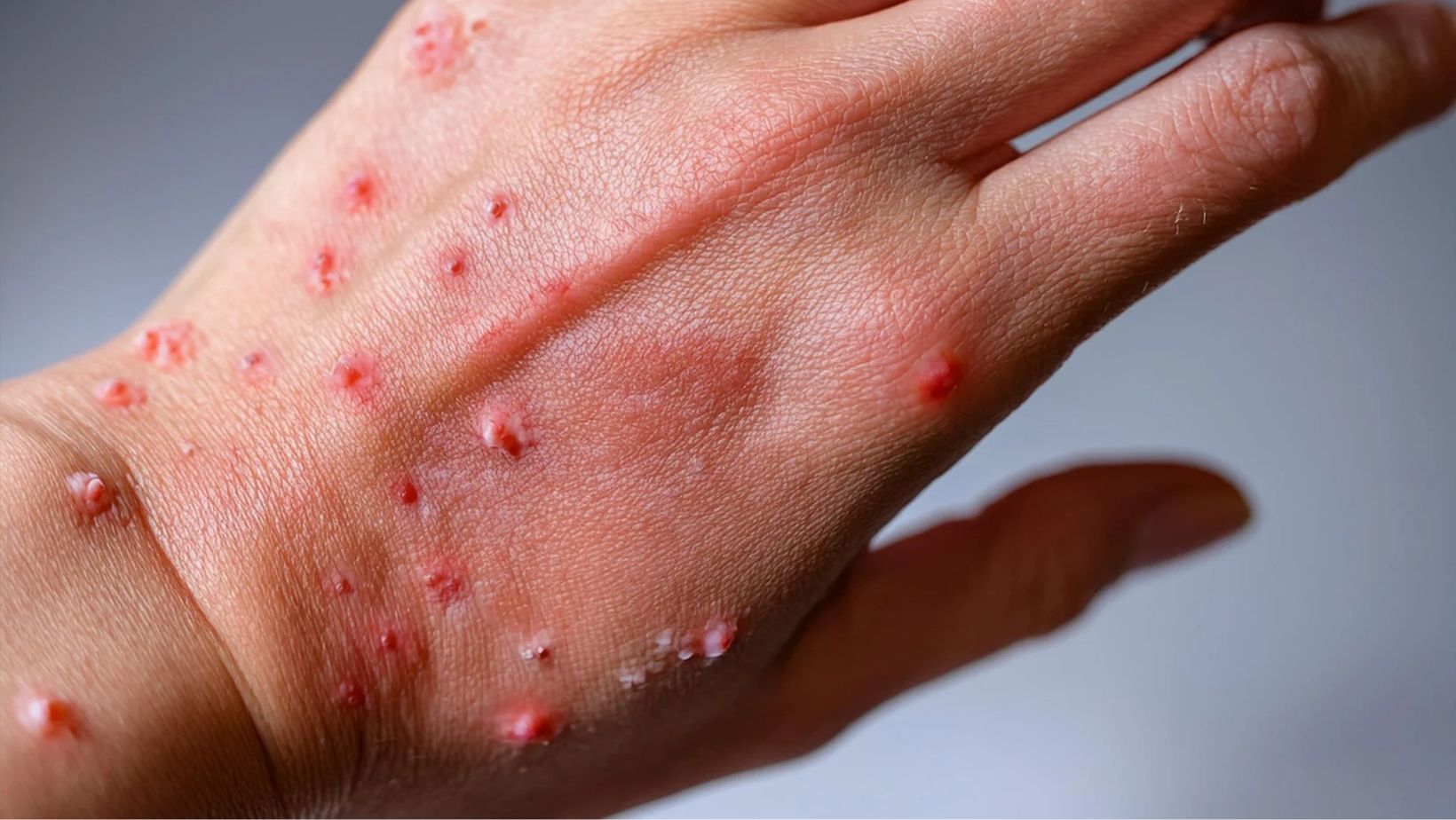
While smallpox was eradicated in 1980 following a global vaccination campaign, research into smallpox vaccines continues due to concerns over bioterrorism. Mpox, a related disease caused by the monkeypox virus, has triggered multiple outbreaks since 2022, with case fatality rates ranging from 0.2% to 11%. The two FDA-approved smallpox vaccines are also authorized for use against pox, but researchers believe a more effective and safer alternative is needed.
Previous studies have shown that the experimental horsebox-based vaccine generated a strong immune response in non-human primates while preventing disease after exposure to the monkeypox virus. Mice given its exhibited no adverse effects, unlike those given the traditional vaccinia-based vaccine, which often led to severe symptoms.
Next Steps in Vaccine Development

Researchers are now preparing for phase I clinical trials to assess the experimental of its safety in humans. If successful, it could provide a more efficient option for protecting against smallpox and pox, reducing the need for multiple doses while lowering the risk of side effects.
Although smallpox remains confined to laboratory settings in the United States and Russia, the potential threat of future pox outbreaks has prioritized its research. Scientists hope this new approach will provide a safer and more practical option for disease prevention.
Reference: Stephanie V. Trefry, Mayanka Awasthi, Christy N. Raney, Amy L. Cregger, Chase A. Gonzales, Brittney L. Layton, Robert N. Enamorado, Nelson A. Martinez, Deborah S. Gohegan, Masoudeh Masoud-Bahnamiri, Jennifer Y. Cho, Dawn M. Myscofski, Tinoush Moulaei, Natasza E. Ziółkowska, Scott J. Goebel, Seth Lederman, Sina Bavari, Farooq Nasar. Recombinant chimeric horsepox virus (TNX-801) is attenuated relative to vaccinia virus strains in both in vitro and in vivo models. mSphere, 2024.

