Researchers at the University of Wisconsin-Madison have unveiled high-resolution images of respiratory syncytial virus (RSV), providing new insights into its structure and potential vulnerabilities. The findings, recently published in Nature, could help pave the way for more effective treatments for an infection that hospitalizes hundreds of thousands of people in the United States annually, according to the Centers for Disease Control and Prevention (CDC).
A Virus with Limited Treatment Options
RSV primarily affects young children, older people, and individuals with underlying respiratory conditions. Despite its significant impact, treatment options remain limited. While prophylactic therapies exist for young children, and vaccines are available for pregnant women and older adults, broader preventive measures have been difficult to develop—mainly due to the virus’s complex structure.
Unlike more easily studied viruses, RSV comprises flexible, filament-like structures that have complicated researchers’ efforts to identify reliable drug targets. The UW-Madison team used cryo-electron tomography (cryo-ET) to address this challenge. This advanced imaging technique freezes biological molecules at ultracold temperatures to capture them in their natural states. This method allows scientists to generate detailed three-dimensional images of viral structures at atomic resolution.
Key Protein Structures Revealed
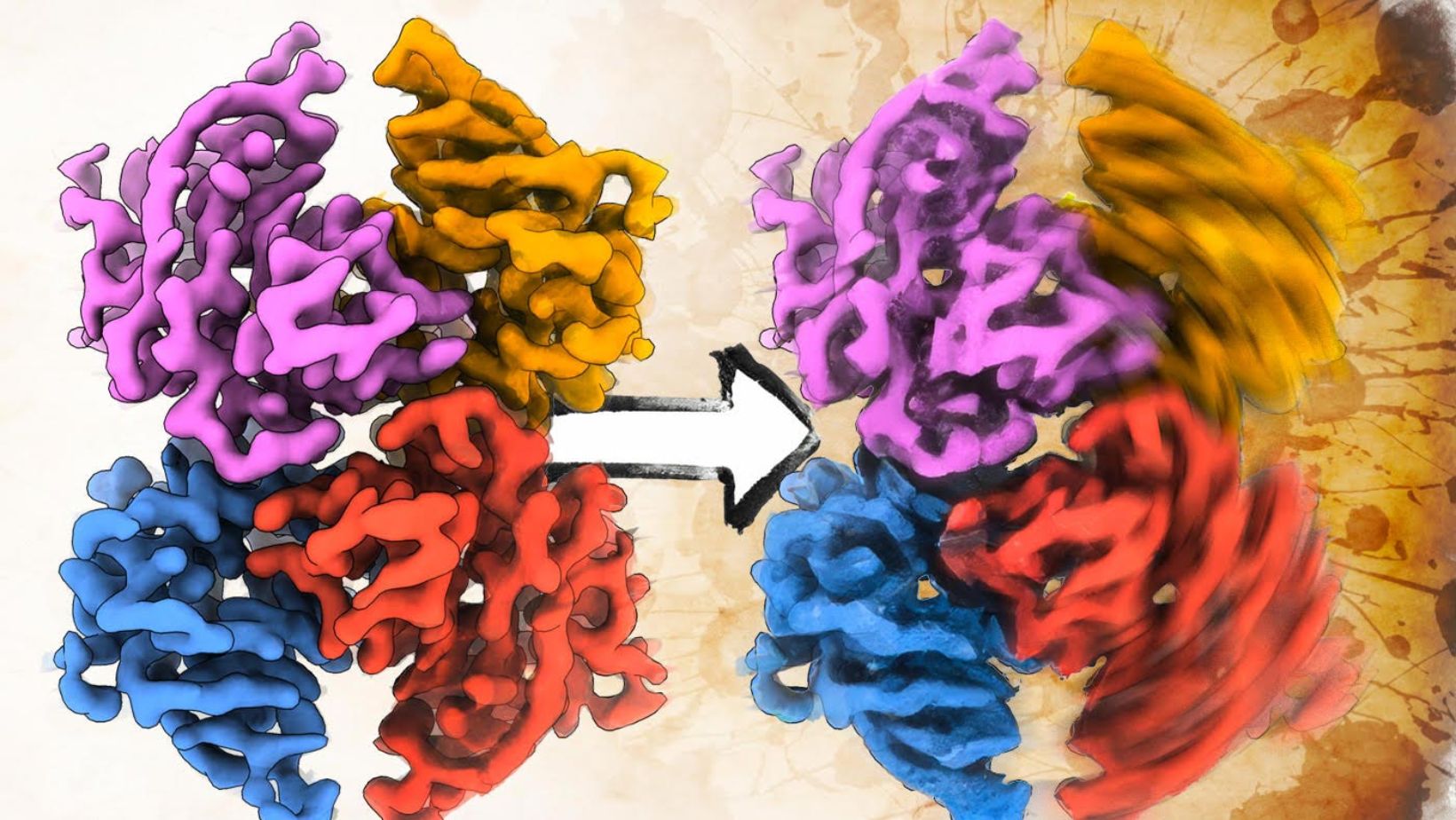
The study focused on two key RSV proteins: the M protein and the F protein. The M protein plays a structural role, helping to maintain the virus’s filamentous form and coordinate viral assembly. The F protein, which sits on the virus’s surface, is essential for initiating infection by binding to host cells and enabling viral entry. The researchers discovered that RSV F proteins form stable pairs, a structural feature that may regulate when and how the virus infects cells.
Elizabeth Wright, a professor of biochemistry at UW-Madison and senior author of the study, emphasized the significance of these findings. “Our primary findings reveal structural details that allow us to understand better not only how the protein regulates assembly of viral particles, but also the coordination of proteins that enable the virus to be infectious,” Wright said.
Potential for Future Treatments
This discovery could have important implications for drug development. If researchers can identify ways to disrupt the F protein pairing process, they may be able to prevent RSV from infecting new host cells. Wright and her team plan to continue investigating these protein interactions to develop targeted treatments to reduce the severity and spread of RSV infection.
Reference: Bryan S. Sibert, Joseph Y. Kim, Jie E. Yang, Zunlong Ke, Christopher C. Stobart, Martin L. Moore, Elizabeth R. Wright. Assembly of respiratory syncytial virus matrix protein lattice and its coordination with fusion glycoprotein trimers. Nature Communications, 2024.

